A new technology called PAGER allows researchers to create custom G-protein-coupled receptors.Credit: Juan Gaertner/Science Photo Library
Cell-surface receptors are crucial for cells to sense and respond to the environment. After binding to a specific chemical outside the cell, the membrane-spanning proteins trigger signalling cascades or new gene transcription, ultimately changing cellular behaviour.
G-protein-coupled receptors (GPCRs) — which make up the largest and most diverse family of receptors in humans — are encoded by at least 800 distinct genes. Many GPCRs have functions in taste, smell and light perception, and manipulating them provides a powerful way for researchers to control cell activity. But doing so often involves a painstaking process of gradually introducing mutations and performing directed evolution in one receptor at a time, meaning the potential of synthetic GPCRs remains largely untapped.
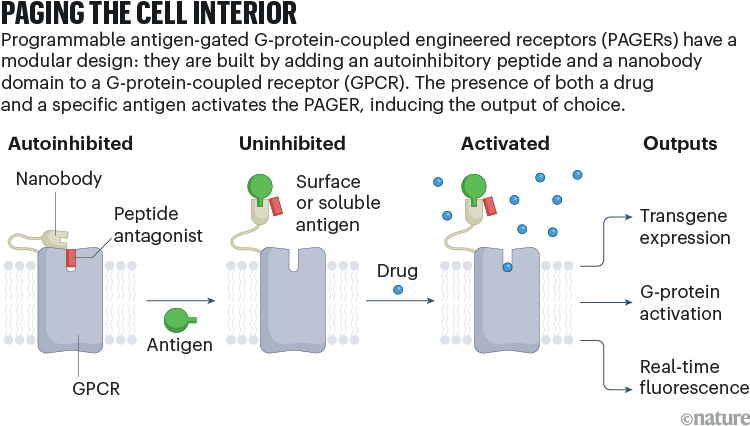
Now, researchers at Stanford University in California and Peking University in Beijing have developed a modular, customizable system for easily creating receptors that can respond to any ligand of interest and produce any of several possible read-outs. The tool, called programmable antigen-gated G-protein-coupled engineered receptors (PAGERs), was described in Nature earlier this month1 (see ‘Paging the cell interior’).
Building on DREADDs
PAGER builds on an existing technology called designer receptors exclusively activated by a designer drug (DREADDs). Developed in 2007 in the laboratory of Bryan Roth, a chemical and synthetic biologist at the University of North Carolina at Chapel Hill, DREADDs are synthetic GPCRs that are activated in cells or transgenic animals only when researchers administer a specific drug. Roth says that they are used widely in neuroscience to “deconstruct brain circuits responsible for behaviour and other things.”
But the PAGER study is the first time that Roth has seen researchers use DREADD to create a new technology. “When I got the paper and I started reading, I said, ‘Holy crap, why didn’t I think of something like this before? This is amazing!’”
To create PAGER, the team — led by chemical biologist Alice Ting — fused two components to the extracellular portion of the DREADD scaffold: an autoinhibitory domain and a nanobody (a tiny antibody fragment) that binds to a target antigen of interest.
The resulting molecule is ‘antigen gated’: in the absence of antigen — which could be a soluble molecule or one fixed on the surface of other cells — the autoinhibitory domain blocks the receptor’s drug-binding pocket, locking it in an ‘off’ state even in the presence of a drug. When an antigen binds to the nanobody, the autoinhibitory domain is displaced and the drug can activate the receptor. Together, these layers of control allow researchers to tune both spatial and temporal responses in cells expressing PAGER proteins.
Among other things, says Josh Leonard, a synthetic biologist at Northwestern University in Evanston, Illinois, the tool could have clinical benefit by allowing researchers to tune the potency of a therapy or mitigate activity in the case of an adverse reaction. “It would be ideal to have the ability to pharmacologically turn such a cell therapy on or off,” he says.
The modularity of the PAGER system stems largely from its nanobody component: because nanobodies for different antigens are of similar size and geometry, researchers can easily swap one nanobody for another, to target any antigen for which a suitable nanobody exists. Ting’s team designed more than a dozen variants, including molecules that could respond to fluorescent proteins, growth factors and cell-surface proteins.
Researchers can also customize the consequences of PAGER activation by selecting a scaffold with the appropriate downstream effector function. The team demonstrated designs that could activate a transgene, initiate a signalling cascade or induce fluorescence as a kind of sensor.
